Periodic Trends Worksheet 2. Pat Ligon Created Date.
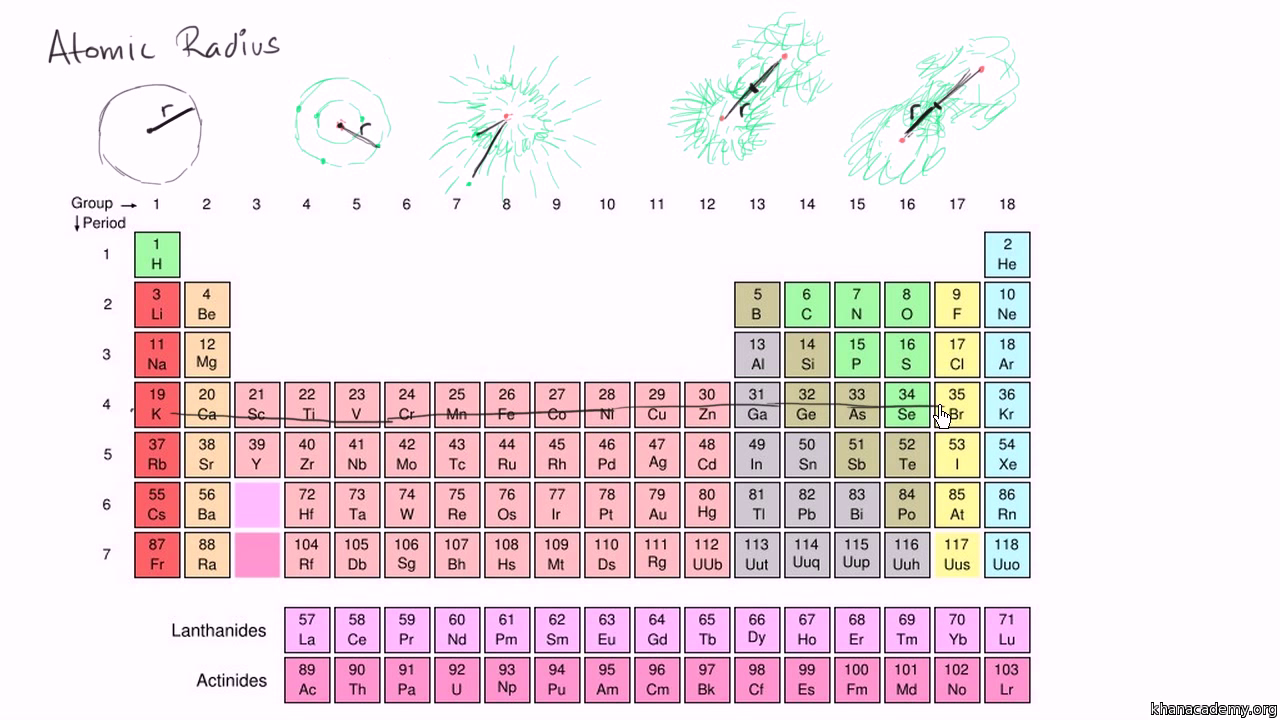
Atomic Radius Trends On Periodic Table Video Khan Academy
Atomic number for Group 2A and for Period 3 of the periodic table.

. Wcpss Last modified by. Os Ni Fe _____ 2. Be able to define the following.
Electronegativity Electron Affinity Ionization Energy Ionic Radius Atomic Radius and Shielding Effect. 3 Why does iodine have a larger radius than fluorine. Periodic Trends Worksheet Name.
Up to 24 cash back Periodic Trends Worksheet Atomic Radius 1. Rank each of the following in order of INCREASING atomic radius a. 3 What is the diffe rence between electron affinity and ionization energy.
Name____Key_____ Date_____Pd_____ Draw the trend for ATOMIC RADIUS. Mg Na Cs _____ c. Mg 2 Ca 2 Ba2 c.
Periodic Trends Worksheet 2 Draw the trend for ATOMIC RADIUS Define Atomic Radius. Students will be able to identify which atoms will have a smallerlarger atomic size and will also be able to explain w. Rank the following elements by increasing electronegativity.
Using your knowledge of Coulombic attraction and the structure of the atom explain the trend in atomic radius that you identifi ed in Question 2. Covalent Bonds assignment answer key. Draw the trend for METALLIC CHARACTER Metallic character.
Not use the word radius in your defi nition. Carbon aluminum o xygen. F K Br _____ Mg Na Cs _____ Os Ni Fe _____.
Opening Prayer - Lecture notes 1. Rank each of the following in order of INCREASING. Al Cl Ga 2.
What causes this. Multiple choice questions for periodic table trends name date periodic trends worksheet directions. Use the Periodic Table and your knowledge of periodic trends to answer the following questions.
Download 18300 B Periodic Trends Q and A. Down the group on the periodic table. Circle the element Cu K Ni Br with the largest atomic radius and put a square around the element with the smallest atomic radius.
Calcium iron neon nitrogen silicon. Periodic Trends Worksheet ATOMIC RADIUS 1. Sulfur oxygen neon aluminum.
Carbon aluminum oxygen potassium. Put the following elements in order from smallest to largest atomic radius. Carbon aluminum oxygen potassium.
Rank the following elements by increasing atomic radius. What trend in atomic radius do you see as you go down a groupfamily on the periodic table. Li Na K c.
Draw the trend for ATOMIC RADIUS. Periodic Trends Worksheet 2 Draw the trend for ATOMIC RADIUS Define Atomic Radius. Below is a rough sketch of the Periodic Table.
A summary worksheet activity of the periodic table trends including. Draw the trend for ATOMIC RADIUS Rank each of the following in order of INCREASING atomic radius Mg Na os M Fe Rank each of the following in order ofDECREASING atomic radius ca Rb C Pb Draw the trend for IONIZATION ENERGY e creases. Cl Br Ga Ga Br Cl b.
Arrows to show increasing periodic trends or decreasing periodic trends. Up to 24 cash back Periodic Trends Worksheet 1 Rank the following elements by increasing atomic radius. A Al B b S O c Br Cl d Na Al e O F f Mg Ca 6.
Electronegativity atomic radius electron affinity ionization energy. Periodic Trends Worksheet 3. Support your answer using examples from three groups.
Circle the atom in each pair that has the largest atomic radius. What trend in atomic radius do you see as you go across a periodrow on the periodic table. Mg 2 Si 4- S 2-b.
Trends of the Periodic Table - Atomic size RadiusThis lesson plan will teach your students about the atomic size radius trend within groups and periods of the Periodic Table. F K Br _____ b. Rank each of the following in order of INCREASING atomic radius.
Periodic Trends Worksheet Author. Sulfur oxygen neon aluminum. Rank each of the following in order of INCREASING atomic radius.
Using the data below make a bar graph of atomic radius vs. What causes this trend. Valence electrons ion atomic radius ionization energy.
View Periodic Trends Worksheet 2docx from CHEM MISC at Santa Maria High School. What causes this trend. Draw the trend for ATOMIC RADIUS.
2 Group 2A Element Atomic Number Atomic Radius Be 4 111 Mg 12 160 Ca 20 197 Sr 38 215 Ba 56 217 Atomic Radius Atomic Number. Make sure you are able to label the group numbers period numbers alkali metals. Bacterial Identification Lab Worksheet Student.
Periodic Trends Worksheet. Use your notes to answer the following questions. What causes this trend.
In general what is the trend in atomic radius as you go down a group in Model 1. FlexBook Platform FlexBook FlexLet and FlexCard are registered trademarks of CK-12 Foundation. IONIC RADIUS For each of the following sets of ions rank them from smallest to largest ionic radius.
Ge P O d. Several interesting questions with important charts and diagrams. Gizmos Eyes Vision 1 - Seeing Color Activity A colors of light Activity B reflected light Activity C mixtures of light.
F K Br FBrK b. Os Ni Fe Ni Fe Os Rank each of the following in order of DECREASING atomic radius a. What causes this trend.
Up to 24 cash back Periodic Trends Worksheet Name_____ Date_____Pd_____ Draw the trend for ATOMIC RADIUS 1. What trend in atomic radius do you see as you go across a periodrow on the periodic table. C N Al e.
Why does fluorine have a. 2 Rank the following elements by increasing electronegativity. Trends-Atomic size Shielding Ionization Energy Electron Affinity.
Stoichiometry Lab Document Joshua Abbott. Use your notes to answer the following questions. March 23rd 2018 - periodic trends worksheet name date pd draw the trend for atomic radius 1 rank each of the following in order of increasing atomic radiusAtomic radius Wikipedia May 2nd 2018 - The atomic radius of a chemical element is a measure of the size of its atoms These trends of the atomic radii drawing the outermost electrons.
1 Rank the following elements by increasing atomic radius. 2 Rank the following elements by increasing electronegativity. What trend in atomic radius occurs do wn a group on the periodic table.
You just need to remember whats happening to z effective which really tells us whats happening with all the trends and once you know z effective you can figure out for example what direction the atomic radius should be going into. F K Br _____ Os Ni Fe _____ Rank each of the following in order of DECREASING atomic radius. Sketch in whether the following increase or decrease going across a period left to right or going up a group.
Use COMPLETE SENTENCES for your answers. Circle the atom in each pair that. Circle the one from each pair that would be the larger in size.
Hesi-mental-health-rn-v1-v3-2020- test bank for 2020-2021. Rank the following elements by increas ing atomic radius. What causes this trend.
Li C F b. Rank each of the following in order of DECREASING atomic radius. Ca Rb C Rb Ca C Circle the atom in each pair that has the largest atomic radius.
Rank each of the following in order. ATOMIC RADIUS For each of the following sets of atoms rank the atoms from smallest to largest atomic radius. Rank each of the following in order of INCREASING atomic radius a.
What trend in atomic radius occurs down a group on the periodic table. Calcium iron neon nitrogen silicon. As we compare the elements down the group the effective nuclear charge increases but at the same time the outermost electrons are found in the shell that is farther away from the nucleus.
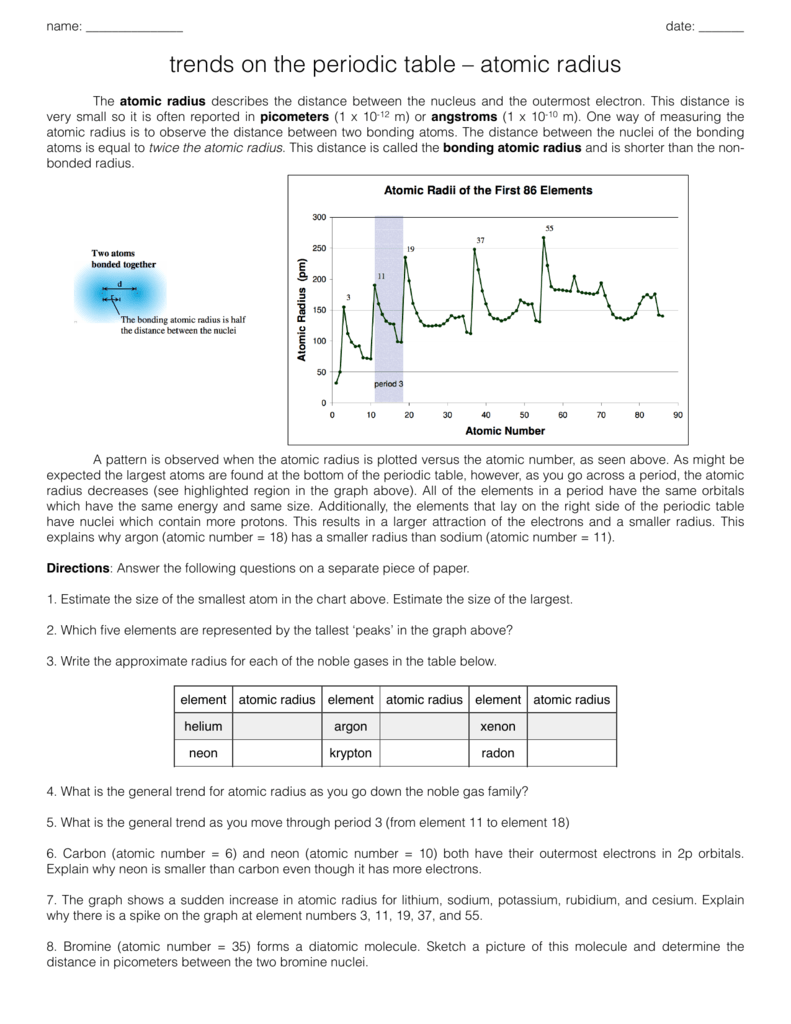
Atomic Radius Practice Problems

Periodic Trends Guided Inquiry Activity Chemical Education Xchange

Periodic Trends Atomic Radius Ck 12 Foundation
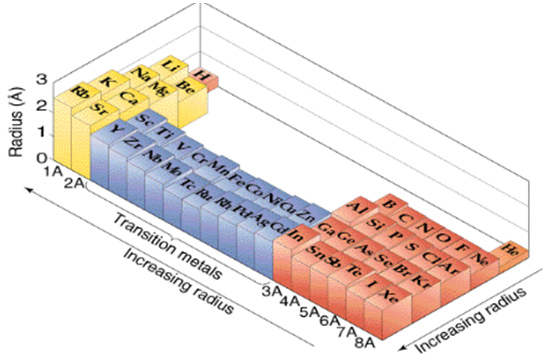
Periodic Table Trends Texas Gateway

Easy To Use Chart Of Periodic Table Trends Electron Affinity Science Notes Chemistry

Periodic Trends Webquest And Graphing Ps1 1 Graphing Ionization Energy Webquest
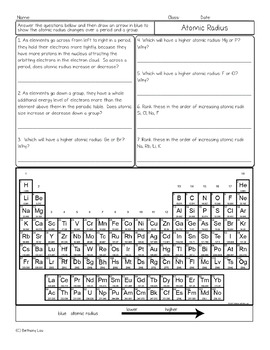
Atomic Radius Periodic Table Trend Chemistry Homework Worksheet Tpt

0 comments
Post a Comment